While it is has long been noted that genome stability is a central function required for survival of stem cells, the role of protein homeostasis has not been explored. With the asymmetric divisions invoked by stem cells, the passage of damaged proteins to daughter cells can destroy the resulting lineage of cells and possibly accelerate the aging process. Furthermore, the possible retention of damaged proteins by the stem cell can result in diminished stem cell function and possibly premature aging. Therefore, a firm understanding of how stem cells maintain their proteome is of central importance. The main hypothesis of our laboratory is that protein quality control is a key determinant of stem cell function that can drive the aging process. Because experiments with mammalian embryonic stem cells have clearly demonstrated their capacity to replicate continuously and the absence of senescence, we hypothesize that these cells could provide a novel paradigm to study the regulation of protein homeostasis and its demise in aging. We have discovered that human embryonic stem cells have an incredibly high proteasome activity compared to many non-stem cell lineages. Furthermore, we have uncovered that PSMD11/rpn-6, a key proteasomal subunit, is required for this activity and its mode of regulation and conservation in the aging process of the invertebrate C. elegans. One of the next big challenges will be to determine how the protein homeostasis network impinges upon stem cell function with an eye to understand how this network can be adapted to alleviate age-related pathologies such as Alzheimer’s, Parkinson’s and Huntington’s disease by using C. elegans and mice as organismal models.
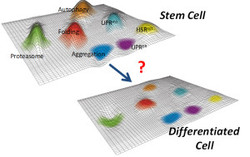
Figure 1: We hypothesize that stem cells could have increased mechanisms to maintain the stability of their proteome. Proteostasis landscape schemes adapted from Douglas & Dillin (2010).
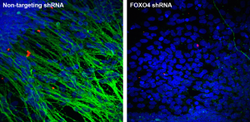
Figure 2: FOXO4, a forkhead transcription factor, regulates proteasome activity in human embryonic stem cells (hESCs) and is necessary for hESCs differentiation into neural cells. Therefore, our results establish a novel regulation of protein homeostasis in hESCs that links longevity and stress resistance in invertebrates with hESC function and identity (Vilchez et al, 2012b, Vilchez et al 2013). Representative images of immunocytochemistry after neural differentiation assay. Neuronal marker: β-III-tubulin, green. Nuclear staining: DAPI, blue Staining: David Vilchez, Leah Boyer.
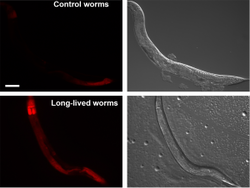
Figure 3: Representative images of RFP expressed under control of the rpn-6 promoter. Rpn-6 expression is increased in the long-lived glp-1(e2141) animals relative to N2 worms. Scale bar, 100 µm (Vilchez et al, 2012a). Ectopic expression of rpn-6 is sufficient to confer proteotoxic stress resistance and extend lifespan, indicating that rpn-6 is a candidate to correct deficiencies in age-related protein homeostasis disorders.